Answer:
The correct answer is 0.206 moles
Step-by-step explanation:
According to the given scenario, the calculation of the number of moles of ammonium chloride is available in the resulting solution is given below:
Given that
Amount of
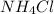 is 11.0 grams
is 11.0 grams
And, the volume is 235 mL
Now the molar mass of
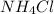 is 53.49g/mol
is 53.49g/mol
So, the number of moles presented is
= 11.0 ÷ 53.49
= 0.206 moles
hence, the number of moles of ammonium chloride are available in the resulting solution is 0.206 moles