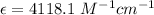Complete Question
49.9 ml of a 0.00292 m stock solution of a certain dye is diluted to 1.00 L. the diluted solution has an absorbance of 0.600. what is the molar absorptivity coefficient of the dye
Answer:
The value is
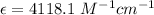
Step-by-step explanation:
From the question we are told that
The volume of the stock solution is
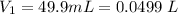
The concentration of the stock solution is
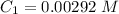
The volume of the diluted solution is
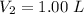
The absorbance is
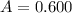
Generally the from the titration equation we have that
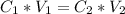
=>
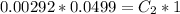
=>
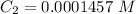
Generally from Beer's law we have that
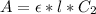
=>
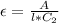
Here l is the length who value is 1 cm because the unit of molar absorptivity coefficient of the dye is

So
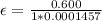
=>