Answer:
T₂ = 280.17 K
Step-by-step explanation:
Here, we can use the equation of state to find the final temperature of air in tires. Equation of State is written as follows:
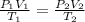
Since, the volume is constant.
Therefore,
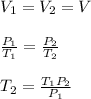
where,
T₂ = Final Temperature of Air in Tire= ?
T₁ = Initial Temperature of Air = 270.734 K
P₁ = Initial Pressure of Air = 454.518 KPa
P₂ = Final Pressure of Air = 470.361 KPa
Therefore,
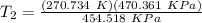
T₂ = 280.17 K