Answer:
0.267 mol
Step-by-step explanation:
The unbalanced equation for the reaction that takes place is:
Once we balance it we're left with:
- 2C₈H₁₈ + 25O₂ → 18H₂O + 16CO₂
Using the stoichiometric ratio of water and octane from the balanced reaction, we can convert mol of water into mol of octane:
- 2.40 mol H₂O *
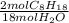 = 0.267 mol C₈H₁₈
= 0.267 mol C₈H₁₈
Thus 0.267 moles of octane are needed to produce 2.40 mol of water.