Answer:
1 mole of Sulfuric Acid
Step-by-step explanation:
Given
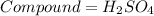
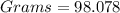
Required
Determine the amount of moles
First, we need to determine the atomic mass of the acid.
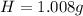 -- Hydrogen
-- Hydrogen
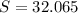 --- Sulfur
--- Sulfur
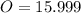 -- Oxygen
-- Oxygen
So:
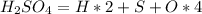
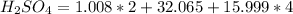
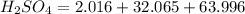
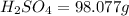
Number of moles (n) is then calculated as thus:
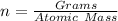
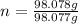
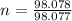
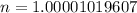
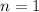 (approximated).
(approximated).
Hence, there is 1 mole of Sulfuric Acid