The balanced equation for this reaction is
 .
.
This means that for every mole of iron(III) oxide consumed, 3 moles of carbon monoxide are consumed.
- Iron has an atomic mass of 55.845 g/mol.
- Oxygen has an atomic mass of 15.999 g/mol.
This means that iron(III) oxide has a formula mass of 2(55.845)+3(15.999)=g/mol, and thus 318 g is equal to
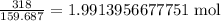 .
.
So, this means we need
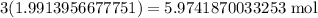 of CO.
of CO.
- Carbon has an atomic mass of 12.011 g/mol.
- Oxygen has an atomic mass of 15.999 g/mol.
This means CO has a formula mass of 12.011+15.999=28.01 g/mol, and thus 5.9741870033253 moles have a mass of (28.01)(5.9741870033253), which is equal to about 167 g (to 3 sf)