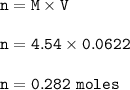Moles of HCl = 0.282
Further explanation
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
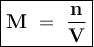
Where
M = Molarity
n = number of moles of solute
V = Volume of solution
The volume of HCl=62.2 ml=0.0622 L
The molarity of HCl = 4.54 M
Then moles of HCl :