Answer:
V₂ = 252 cm³
Explanation:
Charles's law = If the pressure is held constant, the volume of a gas varies directly with the temperature measured on the Kelvin scale.
Mathematically,
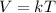
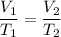
We have, V₁ = 192 cm³, T₁ = 112 K, T₂ = 147 cm³, V₂ = ?
Using the above relation of Charles's law :
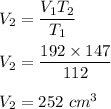
So, the new temperature is 252 cm³.