Answer:
B
Explanation:
First, you need to simplify your equation so that all the parts are on the same side. You do this by subtracting 13 on both sides:
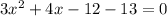
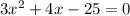
Now, you can plug these values into the quadratic formula. The number with the
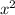 is a, the number with the
is a, the number with the
 is b, and the number without a variable is c. The quadratic equation is as follows:
is b, and the number without a variable is c. The quadratic equation is as follows:
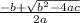
When you plug all these values in, you get answer choice B.
Also note that the equation should be -b ± (plus or minus) but I could not include it in the equation.