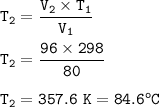The final temperature of the gas : 84.6 °C
Further explanation
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
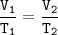
T₁=initial temperature=25°C+273=298 K
V₁=initial volume=80 cm³
volume increment : 20% x 80 cm³=16 cm³
V₂ = Final volume = 80 cm³ + 16 cm³ = 96 cm³
So the final temperature :