Answer:
pH = 14.05.
Step-by-step explanation:
Hello!
In this case, since barium hydroxide ionizes according to the following equation:
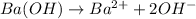
We can compute the concentration of hydroxyl ions based on:
![[OH^-]=0.606(molBa(OH)_2)/(L)*(2molOH^-)/(1molBa(OH)_2) =1.21M](https://img.qammunity.org/2021/formulas/chemistry/college/5jmsw3gkzjf7j35voss928wwupg1uo0c49.png)
Next we compute the pOH:
![pOH=-log([OH^-])=-log(1.12)=-0.0500](https://img.qammunity.org/2021/formulas/chemistry/college/4eqb6e7qmvye33o2vtgoa794dx6uzcdm66.png)
Thus the pH is:
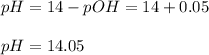
Which means it is very concentrated basic solution.
Best regards!