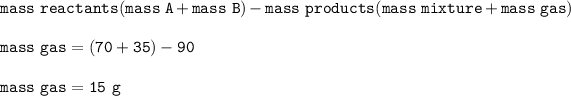Mass of gas produced : 15 g
Further explanation
In general, the reaction takes place in the open air. If the reaction results in the form of gas as in combustion, the mass of the reaction results will be smaller than the original mass.
Conservation of mass applies to a closed system, where the masses before and after the reaction are the same
So In a closed/isolated system, the total mass of the substance before the reaction will be equal to the total mass of the reaction product.
Mass of solution A = 70 g
Mass of solution B = 35 g
Mass of mixture : 90 g
Mass of gas : X
Mass of gas X :