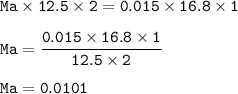The molarity of the aqueous acid solution : 0.0101 M
Further explanation
Titration is a procedure for determining the concentration of a solution by reacting with another solution that is known to be concentrated (usually a standard solution). Determination of the endpoint/equivalence point of the reaction can use indicators according to the appropriate pH range
Acid-base titration formula
Ma. Va. na = Mb. Vb. nb
Ma, Mb = acid base concentration
Va, Vb = acid base volume
na, nb = acid base valence (amount of H⁺, OH⁻)
Va=12.5 ml
na = 2 (H₂SO₄⇒2H⁺+SO₄²⁻⇒2 ion H⁺)
Mb=0.015
Vb=16.8 ml
nb = 1(LiOH⇒Li⁺+OH⁻⇒1 ion OH⁻)
The molarity of the acid(Ma) :