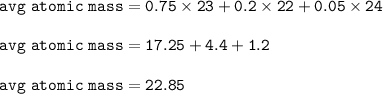The average atomic mass of Sodium : 22.85
Further explanation
The elements in nature have several types of isotopes
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
Mass atom X = mass isotope 1 . % + mass isotope 2.% + ....
isotope 1 : 75%, mass number = 23
Isotope 2 : 20%, mass number = 22
Isotope 3 : 5%, mass number = 24
The average atomic mass :