The daughter isotope (a decay product)of O-15 = N-15(Nitrogen 15)
Further explanation
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
- alpha α particles ₂He⁴
- beta β ₋₁e⁰ particles
- gamma particles γ
- positron particles ₁e⁰
O-15 emits positron particles ₁e⁰, so the atomic number decreases by 1, the mass number is the same
Reaction
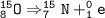
The mass number of the daughter isotope = 15, atomic number = 7
If we look at the periodic system, the element with atomic number 7 is Nitrogen (N)