The pH of the solution : 12
Further explanation
Reaction
HCOOH + NaOH ⇒ HCOONa + H₂O
mol HCOOH =
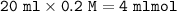
mol NaOH =
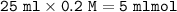
Mol NaOH>mol HCOOH ⇒ at the end of the reaction there will be a strong base remains from mol NaOH, so that the pH is determined from [OH⁻]
ICE method :
HCOOH + NaOH ⇒ HCOONa + H₂O
4 5
4 4 4 4
0 1 1 1
Concentration of [OH⁻] from NaOH :
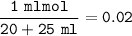
pOH=-log[OH⁻]
pOH=-log 10⁻²=2
pH+pOH=14
pH=14-2=12