Answer:
Choice A.
 electrons would be required for displacing
electrons would be required for displacing
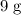 of aluminum from a solution of
of aluminum from a solution of
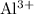 ions.
ions.
Assumption: by "
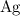 ions" the question meant
ions" the question meant
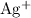 with a charge of
with a charge of
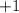 on each ion.
on each ion.
Step-by-step explanation:
The question states that the relative atomic mass of
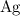 is
is
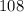 . In other words, each mole of
. In other words, each mole of
Therefore, that
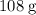 of silver that were formed would contain
of silver that were formed would contain
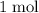 of silver atoms.
of silver atoms.
Metallic silver would precipitate out of this
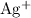 solution only after these ions are turned into
solution only after these ions are turned into
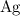 atoms.
atoms.
One
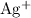 ion carries one unit of positive electrical charge. On the other hand, each
ion carries one unit of positive electrical charge. On the other hand, each
 carries one unit of negative electrical charge.
carries one unit of negative electrical charge.
Therefore, each
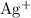 ion will need to gain one electron to form a neutral
ion will need to gain one electron to form a neutral
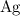 atom.
atom.
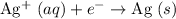 .
.
At least
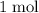 of electrons would be required to turn
of electrons would be required to turn
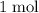 of
of
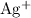 ions into that
ions into that
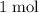 of silver atoms (which have a mass of
of silver atoms (which have a mass of
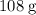 .)
.)
Hence,
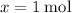 .
.
Unlike
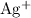 ions, each aluminum ion
ions, each aluminum ion
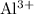 carries three units of positive electrical charge. That is three times the amount of charge on one
carries three units of positive electrical charge. That is three times the amount of charge on one
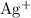 ion. Therefore, three electrons will be required to turn one
ion. Therefore, three electrons will be required to turn one
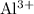 ion to an
ion to an
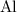 atom.
atom.
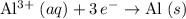
The question states that the relative atomic mass of
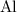 is
is
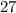 . Therefore, each mole of
. Therefore, each mole of
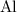 atoms would have a mass
atoms would have a mass
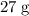 . There would be
. There would be
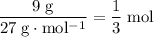 of atoms in that
of atoms in that
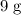 of
of
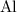 .
.
It takes
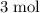 of electrons to turn one mole of
of electrons to turn one mole of
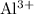 ions to one mole of
ions to one mole of
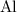 atoms. Hence,
atoms. Hence,
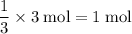 of electrons would be required to produce that
of electrons would be required to produce that
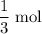 of
of
 atoms (which has a mass of
atoms (which has a mass of
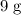 ) from
) from
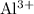 ions.
ions.
That corresponds to the first choice,
 electrons.
electrons.