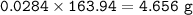The mass of the excess reactant left unreacted : 4.656 g
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
The reaction coefficient in a chemical equation shows the mole ratio of the reactants and products
Reaction
3 CaBr₂ + 2 Na₃PO₄ --> Ca₃(PO₄)₂ + 6NaBr
mass of CaBr₂ = 22.44 g
mol of CaBr₂(MW=199,89 g/mol) :
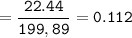
mass of Na₃PO₄ = 16.85 g
mol of Na₃PO₄ (MW=163,94 g/mol) :
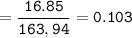
mol ratio of reactants (to find limiring reactant): CaBr₂ : Na₃PO₄ =
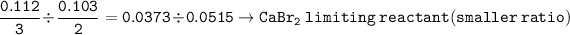
So the excess = Na₃PO₄
mol Na₃PO₄ reacted :
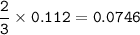
mol Na₃PO₄ unreacted :
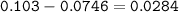
Mass Na₃PO₄ :