The balanced chemical equation :
P₄+3O₂⇒P₄O₆
Further explanation
A compound consists of the mole ratio of the constituent components
12.4 g of phosphorus, mol :
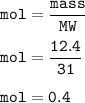
9.6 g of oxygen, mol :
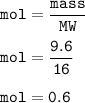
the mole ratio of the compound resulting from the reaction of phosphorus and oxygen :
P : O = 0.4 : 0.6⇒ 4 : 6
The compound formula :
P₄O₆
So the reaction (balanced) :
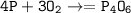
Usually this reaction occurs when phosphorus reacts with a small amount of oxygen instead of excess oxygen(for excess oxygen, the compound produced :P₄O₁₀)