Hey There!
_____________________________________
Answer:
_____________________________________
Balancing the following equations:
(I)
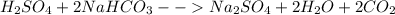
(II)
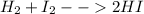
(III)

(IV)
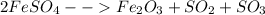
(V)

_____________________________________
Molecular Masses of the following:
------------------------------------------------------------------------------------------------------------
(I)

(ANS)
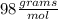
__________________________________________________________
Solution:
Molecular mass of,
H = 1
S = 32
O = 16
Now,
There are 2 atoms of Hydrogen, 1 atoms of Sulfur and 4 atoms of Oxygen present, thus,
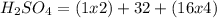
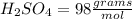
_____________________________________
(II)
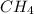
(ANS)
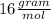
_________________________________________________________
Solution:
Molecular Mass,
Carbon = 12
Hydrogen = 1
Now
There are 1 atom of Oxygen and 4 atoms of Hydrogen present, thus,
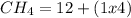
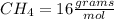
_____________________________________
(III)

(ANS)
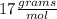
__________________________________________________________
Solution:
Molecular Mass,
Nitrogen = 14 grams
Hydrogen = 1
Now,
There are 1 atoms of Nitrogen and 3 atoms of Hydrogen present, thus,
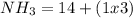
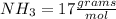
_____________________________________
How many Grams of Iron(fe):

56 71
x 35.5
_____________________________________
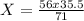
Simplify the equation
X = 28 grams
__________________________________________________________
Answer:
28 grams of Iron(fe) will react with chlorine.
_____________________________________
Best Regards,
'Borz'