It take 0.54 hours to deposit 6.36g of copper
Further explanation
Faraday's Law I
"The mass of the substance formed at each electrode is proportional to the electric current flowing in the electrolysis
W = e.i.t / 96500
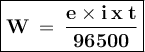
e = equivalent = Ar / valence
i = current, A
t = time, s
W=6.36 g
e = 63.5 : 2 =31.75
i = 10 A
