Answer:
The elements in the reactants are the same as the elements in the products
Step-by-step explanation:
The condition for a balanced equation is as follows :
The number of atoms for each element in reactant = Number of atoms for each element in product.
Total charge on reactant and product must be same.
The example of a balanced equation is:
Reaction of carbon and hydrogen :
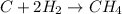
No. of carbon atom on Carbon on LHS = 1
No. of carbon atom on Hydrogen on LHS = 4
No. of carbon atom on Carbon on RHS = 1
No. of carbon atom on Hydrogen on RHS = 4
So, for a balanced equation, The elements in the reactants are the same as the elements in the products. Hence, the correct option (a).