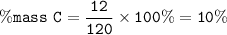The percentage of Carbon (C) present in one molecule of
Dichlorodifluoromethane (CF₂Cl₂) : 10%
Further explanation
Proust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison so that the compound has a fixed composition of elements
With this law, we can calculate how many grams an element is needed to make a compound with a certain mass, as desired
MW Dichlorodifluoromethane (CF₂Cl₂) :
=12+2.19+2.35=120 g/mol