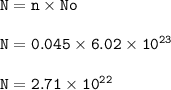Number of molecules in 1 dm³ Oxygen = 2.71 x 10²²
Further explanation
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
The mole is the number of particles contained in a substance
1 mol = 6.02.10²³
1 dm³ of oxygen = 1 L Oxygen
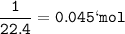
n=mol=0.045
No = 6.02.10²³