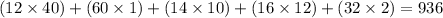Answer:
936g/mol
Step-by-step explanation:
To find the molar mass of a molecule, you find the sum of the molar masses of each atom in the molecule.
This compound has 40 carbon atoms, 60 Hydrogen atoms, 10 Nitrogen atoms, 12 Oxygen atoms, and 2 Sulphur atoms.
The molar mass of:
- Carbon is 12g/mol
- Hydrogen is 1g/mol
- Nitrogen is 14g/mol
- Oxygen is 16g/mol
- Sulphur is 32g/mol