Answer:
A) Sample B has more calcium carbonate molecules
Step-by-step explanation:
M = Molar mass of calcium carbonate = 100.0869 g/mol
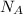 = Avogadro's number =
= Avogadro's number =
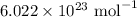
For the 4.12 g sample
Moles of a substance is given by
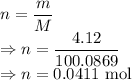
Number of molecules is given by
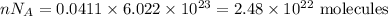
For the 19.37 g sample
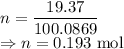
Number of molecules is given by
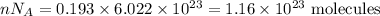
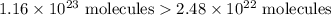
So, sample B has more calcium carbonate molecules.
The ratio of the elements of carbon, oxygen, calcium atoms, ions, has to be same in both the samples otherwise the samples cannot be considered as calcium carbonate. Same is applicable for impurities. If there are impurites then the sample cannot be considered as calcium carbonate.