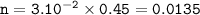Moles HCl reacted : 0.0135
Further explanation
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
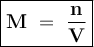
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So to find the number of moles can be expressed as
n = V x M
The volume of HCl 30 cm³=3.10⁻² dm³
Molarity of HCl = 0.45 mol/dm³
so moles HCl reacted :