Hey There!
_____________________________________
Answer:
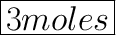
_____________________________________
Compound:
The compound given in the question is
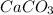 which is called Calcium Carbonate. A 1 mole of Calcium Carbonate has,
which is called Calcium Carbonate. A 1 mole of Calcium Carbonate has,
1 Moles of Calcium.
1 Moles of Carbon.
3 Moles of Oxygen.
_____________________________________
Question:
In the Question, It is asked that how many moles are present in the 1 mole of Calcium Carbonate, So as per information we have, there are 3 moles of oxygen present in Calcium Carbonate.
__________________________________________________________
Much simpler way to understand why there are 3 mole is look at the subscript along the element. There is no number in the subscript of Calcium and Carbon thus 1 Mole, There is 3 present in the subscript thus 3 moles of calcium. Remember that subscript suggests the number of moles of an element in only 1 MOLE OF COMPOUND
_____________________________________
Best Regards,
'Borz'