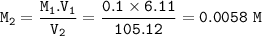Molarity after dilution : 0.0058 M
Further explanation
The number of moles before and after dilution is the same
The dilution formula
M₁V₁=M₂V₂
M₁ = Molarity of the solution before dilution
V₁ = volume of the solution before dilution
M₂ = Molarity of the solution after dilution
V₂ = Molarity volume of the solution after dilution
M₁=0.1 M
V₁=6.11
V₂=105.12