Answer:
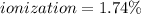
Step-by-step explanation:
Hello!
In this case, since the degree of ionization of an acid is computed in terms of the concentration of hydrogen ions and the initial concentration of the acid:
![ionization=([H^+])/([HA]) *100\%](https://img.qammunity.org/2021/formulas/chemistry/college/1zhzpvq9sa3bvrofgqwp01cb7qtmgyo7ae.png)
Because the ionization reaction is represented by:
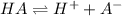
Therefore, the degree or percent ionization turns out:
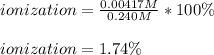
Best regards!