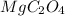Answer:
Compound x =
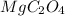
Step-by-step explanation:
Let the compound be x
Assuming we have a 100g of compound x
Given the following data;
Magnesium, Mg = 21.6% = 21.6g
Carbon, C = 21.4% = 21.4g
Oxygen, O = 57.0% = 57.0g
Now, we would find the amount of moles for each element.
Atomic mass of Mg = 24.30g
Atomic mass of C = 12.01g
Atomic mass of O = 16.00g
Amount of moles for Mg;
21.6*(1/24.30) = 0.89mol
Amount of moles for C;
21.4*(1/12.01) = 1.78mol
Amount of moles for O;
57.0*(1/16.00) = 3.56mol
We then divide by the smallest to find the ratio;
0.89/0.89 = 1 Mol of Mg
1.78/0.89 = 2 Mol of C
3.56/0.89 = 4 Mol of O
Therefore, the ratio of Mg, C and O is 1:2:4.
Compound x =
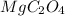
Hence, the empirical formula of the compound is