pH=4.625
The classification of this sample of saliva : acid
Further explanation
The water equilibrium constant (Kw) is the product of concentration
the ions:
Kw = [H₃O⁺] [OH⁻]
Kw value at 25° C = 10⁻¹⁴
It is known [OH-] = 4.22 x 10⁻¹⁰ M
then the concentration of H₃O⁺:
![\tt 10^(-14)=4.22* 10^(-10)* [H_3O^+]\\\\(H_3O^+]=(10^(-14))/(4.22* 10^(-10))=2.37* 10^(-5)](https://img.qammunity.org/2021/formulas/chemistry/high-school/3t72bx94ckktsz48b3ylg0pk7t5327wd0l.png)
pH=-log[H₃O⁺]
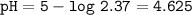
Saliva⇒acid(pH<7)