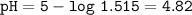a.NH₃+HCl⇒NH₄Cl
b.volume HCl=150 ml
c. pH=4.82
Further explanation
Reaction
NH₃+HCl⇒NH₄Cl
The equivalence point⇒mol NH₃=HCl
Titration formula :
M₁V₁n₁=M₂V₂n₂(n=acid base valence, NH₃=HCl=1)
mol NH₃
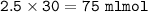
mol HCl=75 mlmol
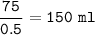
Volume total :
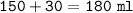
mol NH₄Cl=mol NH₃=75 mlmol=0.075 mol
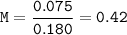
Dissociation of NH₄Cl at water to find [H₃O⁺]
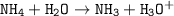
ICE at equilibrium :
0.41-x x x
Ka(Kw:Kb)= 10⁻¹⁴ : 1.8.10⁻⁵=5.6.10⁻¹⁰
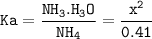
[H₃O⁺]=x :
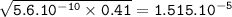
pH=-log[H₃O⁺]