Answer:
B
Step-by-step explanation:
Recall the ideal gas law:
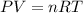
Because pressure and amount is kept constant while volume and temperature changes, we can rearrange the formula as:
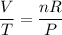
The right-hand side is simply some constant. In other words:
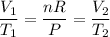
Substitute in known values and solve:
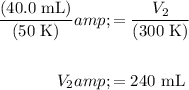
In conclusion, our answer is B.