Answer:
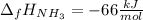
Step-by-step explanation:
Hello!
In this case, since the study of the bond energy allows us to compute the enthalpies of some reactions, for this combination reaction by which ammonia is yielded, we understand the enthalpy of reaction equals the enthalpy of formation of ammonia, and, in terms of the bonds energy we can write:
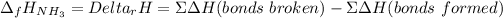
Whereas the bonds enthalpy of those bonds that get broken cover the N≡N and the three H-H bonds at the reactants side and the enthalpy of those bonds that are formed cover the six N-H bonds at the products; which means we obtain:
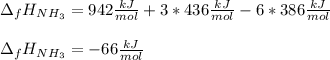
Which differs from the theoretical value that is -46 kJ/mol.
Best regards!