Answer:
22.366 kPa
Step-by-step explanation:
The final pressure can be found by using the formula for Boyle's law which is
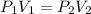
Since we are finding the final pressure
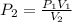
From the question
58.6 kPa = 58600 Pa
We have
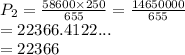
We have the final answer as
22.366 kPa
Hope this helps you