Answer:
ka = 2x10⁻⁹
Step-by-step explanation:
Using the pH we can calculate the molar concentration of H⁺, [H⁺]
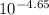 = [H⁺] = 2.24 x 10⁻⁵ M
= [H⁺] = 2.24 x 10⁻⁵ M
Then we use the expression of Ka for the equilibrium of a weak monoprotic acid:
HA ↔ H⁺ + A⁻
Where:
ka =
![([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/jf8hqw697l8ucn8pfdec399k3qomj6l723.png)
ka =
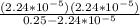 = 2x10⁻⁹
= 2x10⁻⁹