Step-by-step explanation:
1.
Given parameters:
Frequency of the radiation = 8.4 x 10¹⁴Hz
Unknown:
Energy of the wave = ?
Solution:
The energy of a wave is given by the expression below;
E = hf
E is the energy
h is the Planck's constant = 6.63 x 10⁻³⁴m²kg/s
f is the frequency
Now insert the parameters and solve;
E = 6.63 x 10⁻³⁴m²kg/s x 8.4 x 10¹⁴Hz
E = 5.57 x 10¹ x 10⁻²⁰J
E = 5.57 x 10⁻¹⁹J
2.
Given parameters:
Wavelength = 2.13 x 10⁻¹³m
Unknown:
Frequency of the wave = ?
Solution:
The frequency of a wave can be determined using the expression;
C = f∧
C is the speed of light = 3 x 10⁸m/s
f is the frequency
∧ is the wavelength
f =
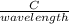 =
=
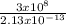 = 1.41 x 10²¹hz
= 1.41 x 10²¹hz