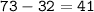Number of neutrons=41, and it is metalloid
Further explanation
Group 14⇒valence electron = 4(ns²np²)
Period 4⇒n=4
So electron configuration of the element :
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p² = 32 electron=atomic number
The element with atomic number 32, which is in period 4 and group 14 is Ge-Germanium
There are seven elements that can be classified as metalloids, namely boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po).
While the mass number: 73
So the number of neutrons = mass number-atomic number