Answer:
D. 313 kJ.
Step-by-step explanation:
Hello!
In this case, since the latent heat of a substance is that heat it releases or absorbs during a phase transition among solid, liquid and gas phases, it is given that it is computed as shown below:
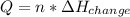
Whereas n are the moles of the substance and the ΔH of change is referred to freezing, melting, condensation, vaporization, sublimation or deposition; therefore, for the vaporization of those gien 7.72 mol of liquid water we obtain:
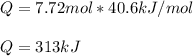
So the answer is D. 313 kJ.
Best regards!