Answer:
Approximately
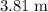 .
.
Step-by-step explanation:
Look up the density
 of carbon tetrachloride,
of carbon tetrachloride,
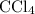 , and glycerol:
, and glycerol:
- Density of carbon tetrachloride: approximately
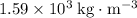 .
. - Density of glycerol: approximately
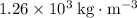 .
.
Let
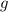 denote the gravitational field strength. (Typically
denote the gravitational field strength. (Typically
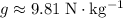 near the surface of the earth.) For a column of liquid with a height of
near the surface of the earth.) For a column of liquid with a height of
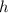 , if the density of the liquid is
, if the density of the liquid is
 , the pressure at the bottom of the column would be:
, the pressure at the bottom of the column would be:
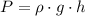 .
.
The pressure at the bottom of this carbon tetrachloride column would be:
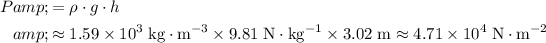 .
.
Rearrange the equation
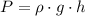 for
for
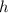 :
:
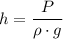 .
.
Apply this equation to calculate the height of the liquid glycerol column:
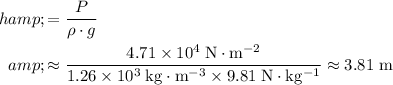 .
.