A. Convert the 200 mol of water to kilograms of water.
Further explanation
Molality (m) shows the number of moles dissolved in every 1000 grams(1 kg) of solvent.
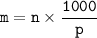
m = Molality
n = number of moles of solute
p = Solvent mass (gram)
or
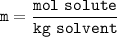
solute = 10 mol NaCl
solvent = 200 mol water⇒Convert to kilograms of water.