Answer:
The final temperature of the bullets is 327.3 ºC.
Step-by-step explanation:
Let suppose that a phase change does not occur during collision and collided bullets stop at the end. We represent the phenomenon by the First Law of Thermodynamics:
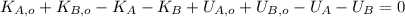 (1)
(1)
Where:
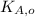 ,
,
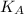 - Initial and final translational kinetic energies of the 15-g bullet, measured in joules.
- Initial and final translational kinetic energies of the 15-g bullet, measured in joules.
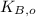 ,
,
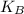 - Initial and final translational kinetic energies of the 7.75-g bullet, measured in joules.
- Initial and final translational kinetic energies of the 7.75-g bullet, measured in joules.
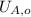 ,
,
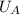 - Initial and final internal energies of the 15-g bullet, measured in joules.
- Initial and final internal energies of the 15-g bullet, measured in joules.
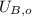 ,
,
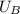 - Initial and final internal energies of the 7.75-g bullet, measured in joules.
- Initial and final internal energies of the 7.75-g bullet, measured in joules.
By definitions of translational kinetic energy and sensible heat we expand and simplify the equation above:
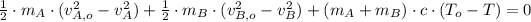 (2)
(2)
Where:
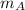 ,
,
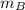 - Masses of the 15-g and 7.75-g bullets, measured in kilograms.
- Masses of the 15-g and 7.75-g bullets, measured in kilograms.
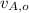 ,
,
 - Initial and final speeds of the 15-g bullet, measured in meters per second.
- Initial and final speeds of the 15-g bullet, measured in meters per second.
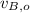 ,
,
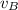 - Initial and final speeds of the 7.75-g bullet, measured in meters per second.
- Initial and final speeds of the 7.75-g bullet, measured in meters per second.
 - Specific heat of lead, measured in joules per kilogram-Celsius degree.
- Specific heat of lead, measured in joules per kilogram-Celsius degree.
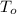 ,
,
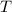 - Initial and final temperatures of the bullets, measured in Celsius degree.
- Initial and final temperatures of the bullets, measured in Celsius degree.
Now we clear the final temperature of the bullets:
![(m_(A)+m_(B))\cdot c \cdot (T-T_(o)) = (1)/(2)\cdot [m_(A)\cdot (v_(A,o)^(2)-v_(A)^(2))+m_(B)\cdot (v_(B,o)^(2)-v_(B)^(2))]](https://img.qammunity.org/2021/formulas/physics/college/ftbl9hpo8f287yh6bltok82qqh5cdd3tbk.png)
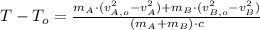
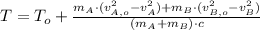 (3)
(3)
If we know that
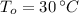 ,
,
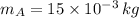 ,
,
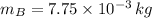 ,
,
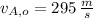 ,
,
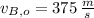 ,
,
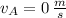 ,
,
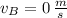 and
and
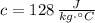 , then the final temperature of the collided bullets is:
, then the final temperature of the collided bullets is:
![T = 30\,^(\circ)C+((15* 10^(-3)\,kg)\cdot \left[\left(295\,(m)/(s) \right)^(2)-\left(0\,(m)/(s) \right)^(2)\right]+(7.75* 10^(-3)\,kg)\cdot \left[\left(375\,(m)/(s) \right)^(2)-\left(0\,(m)/(s) \right)^(2)\right])/((15* 10^(-3)\,kg+7.75* 10^(-3)\,kg)\cdot \left(128\,(J)/(kg\cdot ^(\circ)C) \right))](https://img.qammunity.org/2021/formulas/physics/college/iglqeinj2qstffmola4a3qoeb9yj1puc3c.png)
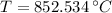
Given that found temperature is greater than melting point, then we conclude that supposition was false. If we add the component of latent heat of fussion, then the resulting equation is:
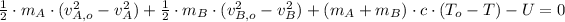 (4)
(4)
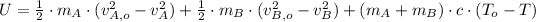
If we know that
 ,
,
 ,
,
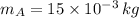 ,
,
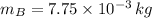 ,
,
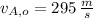 ,
,
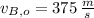 ,
,
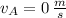 ,
,
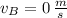 and
and
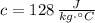 , then latent heat received by the bullets during impact is:
, then latent heat received by the bullets during impact is:
![U =(1)/(2)\cdot (15* 10^(-3)\,kg)\cdot \left[\left(295\,(m)/(s) \right)^(2)-\left(0\,(m)/(s) \right)^(2)\right] + (1)/(2)\cdot (7.75* 10^(-3)\,kg)\cdot \left[\left(375\,(m)/(s) \right)^(2)-\left(0\,(m)/(s) \right)^(2)\right]+(15* 10^(-3)\,kg+7.75* 10^(-3)\,kg)\cdot \left(128\,(J)/(kg\cdot ^(\circ)C) \right) \cdot (30\,^(\circ)C-327.3\,^(\circ)C)](https://img.qammunity.org/2021/formulas/physics/college/9ihpcpngdkuhgrouikf6evtci09qldxzgw.png)
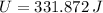
The maximum possible latent heat (
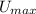 ), measured in joules, that both bullets can receive during collision is:
), measured in joules, that both bullets can receive during collision is:
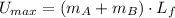 (5)
(5)
Where
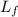 is the latent heat of fusion of lead, measured in joules per kilogram.
is the latent heat of fusion of lead, measured in joules per kilogram.
If we know that
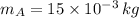 ,
,
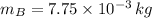 and
and
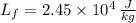 , then the maximum possible latent heat is:
, then the maximum possible latent heat is:
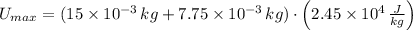
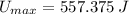
Given that
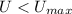 , the final temperature of the bullets is 327.3 ºC.
, the final temperature of the bullets is 327.3 ºC.