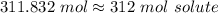Answer:
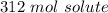
General Formulas and Concepts:
Chemistry
- Molarity = moles of solute / liters of solution
Step-by-step explanation:
Step 1: Define variables
M = 0.722
L = 431.9
m = x
Step 2: Solve for x
- Substitute:
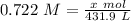
- Isolate x:
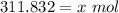
- Rewrite:
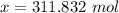
Step 3: Check
Our smallest sig figs is 3. Follow sig figs rules.