Answer:
Throughout the overview section following portion, the description and according to particular circumstance is defined.
Step-by-step explanation:
As per the question,
⇒
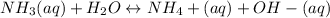
- A weak basis seems to be NH3. It serves as a base since the aqueous solution or phase is protonated. But NH3 +, just becoming a weak base, is therefore deprotonated into form NH3, and therefore also 90% of ammonia becomes found throughout NH3 state in aqueous solution.
⇒
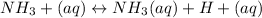
However, it is also available in NH3 form throughout the aqueous solution much of the moment.