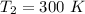Answer:
The temperature of the Nitrogen after throttling is
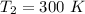
Step-by-step explanation:
From the question we are told that
The temperature is
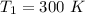
The pressure is
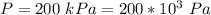
The pressure after being
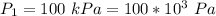
Generally from the first law of thermodynamics we have that
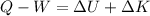
Here
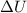 is the change internal energy which is mathematically represented as
is the change internal energy which is mathematically represented as
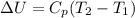
Here
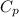 is the specific heat of the gas at constant pressure
is the specific heat of the gas at constant pressure
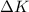 is the change kinetic energy which is negligible
is the change kinetic energy which is negligible
Q is the thermal energy which is Zero for an adiabatic process
W is the work done and the value is zero given that the gas was throttled adiabatically
So
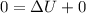
=>
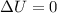
=>
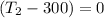
=>