Answer:
Step-by-step explanation:
Given that,
The wavelength of a microwave is 7.42 mm or 0.00742 m
No. of photons, n = 359
We need to find the energy produced by this no of photons. It can be given by the formula as follows :
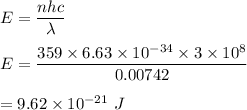
or
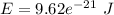
So, the required energy is
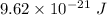 .
.