Answer:
The lava flow is approximately 351.925 million years.
Step-by-step explanation:
We find that atoms of Uranium-235 decays and turns into Lead-207 and that half-life of the former one is
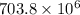 years. The initial amount of Uranium-235 is the sum of current atoms of Uranium-235 and Lead-207. The decay of isotopes is modelled by the following ordinary differential equation:
years. The initial amount of Uranium-235 is the sum of current atoms of Uranium-235 and Lead-207. The decay of isotopes is modelled by the following ordinary differential equation:
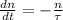 (Eq. 1)
(Eq. 1)
Where:
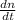 - Rate of change of the amount of Uranium-235 atoms, measured in atoms per year.
- Rate of change of the amount of Uranium-235 atoms, measured in atoms per year.
 - Current amount of Uranium-235 atoms, measured in atoms.
- Current amount of Uranium-235 atoms, measured in atoms.
 - Time constant, measured in years.
- Time constant, measured in years.
The solution of this differential equation is described below:
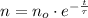 (Eq. 2)
(Eq. 2)
Where:
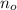 - Initial amount of Uranium-235 atoms, measured in atoms.
- Initial amount of Uranium-235 atoms, measured in atoms.
 - Time, measured in years.
- Time, measured in years.
In addition, we can calculate the time constant in terms of the half-life:
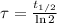 (Eq. 3)
(Eq. 3)
If we know that
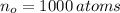 ,
,
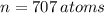 and
and
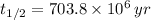 , then the age of the lava flow is:
, then the age of the lava flow is:
From (Eq. 2):
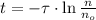
By (Eq. 3);
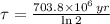
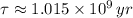
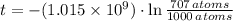
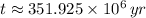
The lava flow is approximately 351.925 million years.