Answer:
0.4941, 0.5059
Step-by-step explanation:
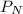 = Partial pressure of nitrogen = 417 mm Hg
= Partial pressure of nitrogen = 417 mm Hg
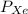 = Partial pressure of xenon = 427 mm Hg
= Partial pressure of xenon = 427 mm Hg
Total pressure in the system is given by
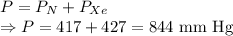
Mole fraction is given by
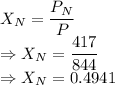
For xenon
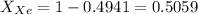
or
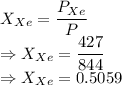
So, mole fraction of nitrogen is 0.4941 and xenon is 0.5059.