Answer:
a. Approximately
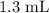 .
.
b. Approximately
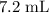 .
.
Step-by-step explanation:
The unit of concentration "
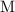 " is equivalent to "
" is equivalent to "
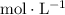 ", which means "moles per liter."
", which means "moles per liter."
However, the volume of both solutions were given in mililiters
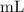 . Convert these volumes to liters:
. Convert these volumes to liters:
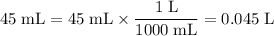 .
.
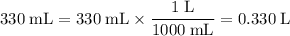 .
.
In a solution of volume
 where the concentration of a solute is
where the concentration of a solute is
 , there would be
, there would be
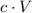 (moles of) formula units of this solute.
(moles of) formula units of this solute.
Calculate the number of moles of
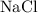 formula units in each of the two solutions:
formula units in each of the two solutions:
Solution in a.:
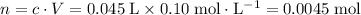 .
.
Solution in b.:
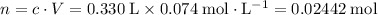 .
.
What volume of that
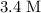 (same as
(same as
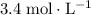 )
)
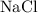 solution would contain that many
solution would contain that many
For the solution in a.:
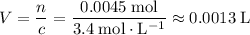 .
.
Convert the unit of that volume to milliliters:
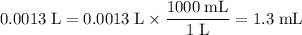 .
.
Similarly, for the solution in b.:
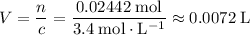 .
.
Convert the unit of that volume to milliliters:
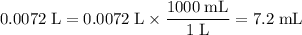 .
.